LabLab



Medipan GmbH is a continuously growing company, in the domestic and international diagnostic markets. Today, the company is an innovative and world renowned partner for laboratories and physicians, for the indications of thyroid function, disease and tumor markers, diabetes and also in the fields of rheumatic and neurological disease.
In 2009, Medipan GmbH became the first company in the world to introduce a commercially available system for the fully automated, objective evaluation of cell-based immunofluorescence tests. The AKLIDES® system marked a new milestone in the development of the immunofluorescence method – one of the oldest and most widespread laboratory procedures for the diagnosis of autoimmune disease. The now-possible standardization and mathematically proven prediction of antibody concentration were the reasons that the system has received several awards. The akiron® NEO is the smaller version of the AKLIDES® for fully automated and standardized image acquisition and data processing in immunofluorescence (AKLIDES® ANA, ANCA and CytoBead® assays).

StressMarq Biosciences is a bioreagents company producing high quality, cutting edge research products for the life sciences and discovery research markets. Our diverse portfolio of antibodies, proteins, kits and small molecules bridges across a spectrum of biomedical research fields including neuroscience, cancer research and cell signaling.
More recently, StressMarq has become a market leader in the development and commercialization of a range of fibrilized and oligomeric protein preparations for use in neurodegenerative disease research, including proteins such as alpha synuclein, tau, SOD1 and TTR. StressMarq’s strategic efforts are currently focused on expanding the breadth and depth of this product offering, including additional fibrilized proteins (and their derivatives), protein oligomers, and antibodies to these constructs to assist researchers studying Alzheimer’s, Parkinson’s, and other neurodegenerative diseases.

Ready-to-use for common systems, e.g. ABBOTT ARCHITECT®, DiaSorin LIAISON®, Roche Elecsys® and others
– vials developed according to the platform
– very small dead volume
– multimarker controls
– barcoded controls
– easy handling
– time saving and cost reducing

Biomatik is here to help make science easy and affordable! We have been proudly serving 10,000+ scientists since 2002, by offering high quality integrated products and services for life sciences research and drug discovery.
Offered Product Lines:
– 38,000+ ELISA Kits
– 14,000+ Antibodies
– 20,000+ Proteins
– 270+ Biochemicals

Ansh Labs’ core immunoassays are novel research clinical diagnostic markers with potential applications in reproductive medicine, neurodegenerative disorders, oncology, etc. Our products consist of emerging and maturing assays that are used by translational medicine and preclinical biomarker testing services, as well as clinical labs around the world.

The Swiss-based TECOmedical Group with subsidiaries in Germany, Benelux and Austria is a leading provider of in-vitro speciality test systems in the areas of medical and veterinary diagnostic, biosafety and environmental testing.

SphingoTec develops and markets innovative in vitro diagnostic (IVD) tests for novel and proprietary blood-based protein biomarkers. These tests allow for the diagnosis, outcome prediction, and monitoring of acute medical conditions, such as septic shock, circulatory failure, acute heart failure and acute kidney injury. SphingoTec’s first-in-class tests are made available on its proprietary whole-blood point-of-care Nexus IB10 platform for convenient and rapid testing in near-patient and laboratory settings alongside a broad portfolio of standard-of-care test for acute care. For high throughput testing in central laboratories, SphingoTec is open to partner with global IVD players.

Accuracy Matters in Salivary Bioscience.
Delivering the most accurate testing results for biomarkers in saliva samples requires reliable collection methods, proper storage and handling techniques, a high-quality assay, and validated testing protocols.
Salimetrics’ role as a leader in standardizing salivary bioscience testing methods allows researchers to deliver the best results and drive research forward.

RSR is a major developer and manufacturer of medical diagnostics with particular emphasis on autoimmune thyroid disease, type 1 diabetes mellitus, neuroimmunology and adrenal autoimmunity. These products are concerned with detection and measurement of various autoantibodies and as such are used in disease diagnosis and management. A high proportion of the company’s output is exported with the European Union, the USA, Japan and China being the main overseas markets.
RSR has an extensive research programme which includes identifying new autoantigens and autoantibodies, understanding the nature of the interaction between autoantigens and autoantibodies and using this information to establish and commercialise new assay methods.

Quidel SPG is the option for all your research, biosafety and cytotoxicity testing needs. Quidel MicroVue products are a well-established name in immune system monitoring, assays for the assessment of Complement activation, as well as biochemical bone markers. Quidel offers products to meet biosafety testing and Complement dependent cytotoxicity testing needs, such as normal human sera, cobra venom factor, and Complement fragment assays. ELISA assays, depleted sera, proteins, monoclonal and polyclonal antibodies, antisera, antigens, controls, and special reagents are also available to round-out a comprehensive laboratory portfolio.

Pathway Diagnostics also manufacturers its own range of haemostasis tests and allergy, autoimmune, tryptase and calprotectin controls that are marketed internationally, together with those of Unicorn Diagnostics Ltd, through local distributors or direct from the UK. The Company also operates its independent PathQAS Quality Assurance schemes (PathQAS) for Coagulation, Allergy, Autoimmune serology, Calprotectin and Prekallikrein Activator assays, as a service to its customers.

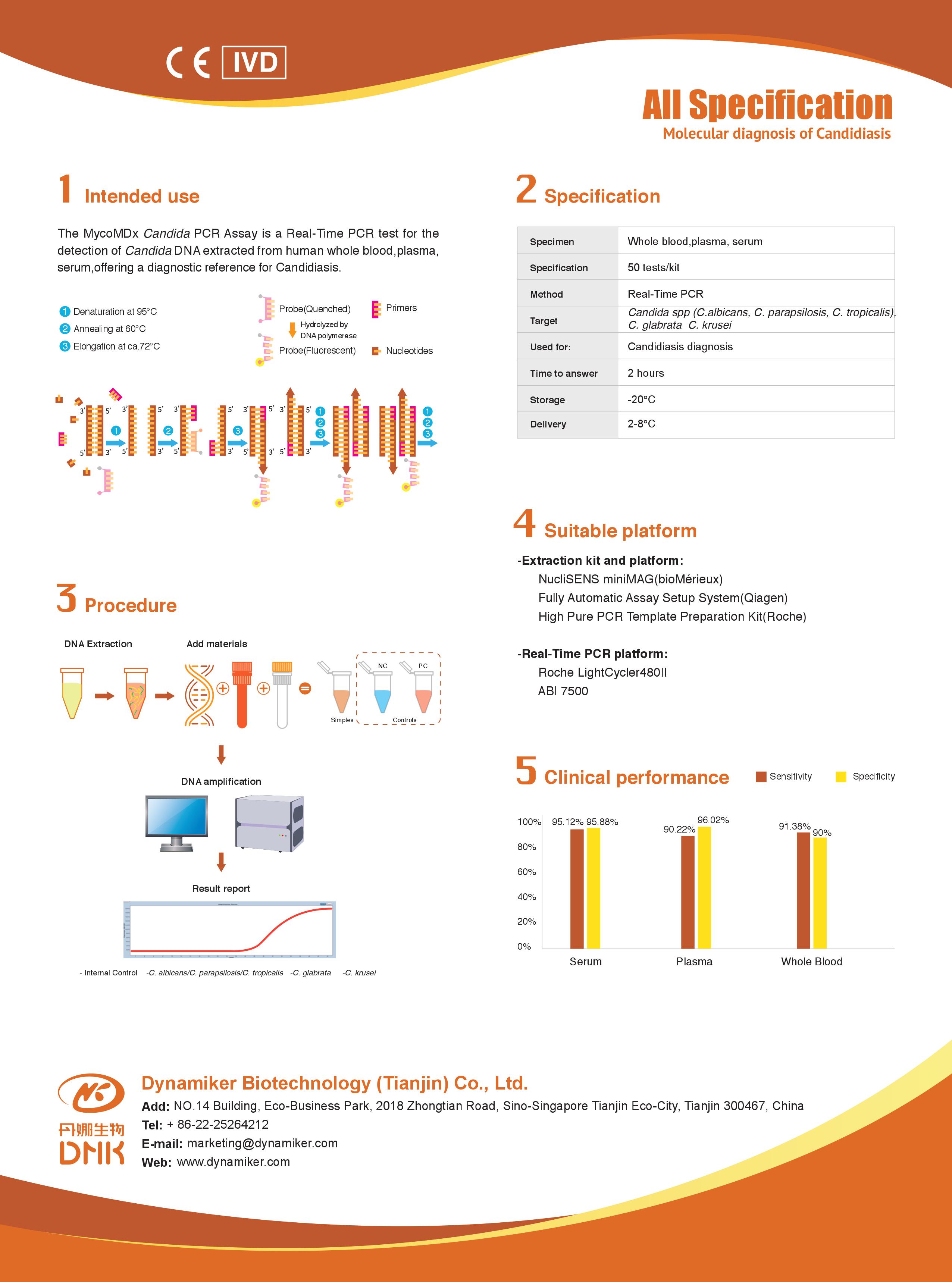
PathoNostics was founded in 2011 in Maastricht, The Netherlands and became an important player in the fungal diagnostic field. PathoNostics is focussing on the development and commercialization of real-time PCR kits for the diagnosis of superficial and invasive fungal infections. PathoNostics currently has four products on the market: AsperGenius®, DermaGenius®, PneumoGenius® and MucorGenius®.

PathoFinder is dedicated to the development, validation and production of high quality, sensitive and robust single reaction multiplex assays, for the detection of pathogens causing an infectious disease. Our products are compatible with many commercially available detection systems to enable a rapid introduction into molecular diagnostics laboratories. PathoFinder will continue to develop new assays for several panel diseases such as Respiratory Tract Infections (RTI’s), Gastroenteritis, Central Nervous System Infections, Sexually Transmitted Diseases (STD’s) and detection of Resistance markers.

OPTOLANE has developed a new diagnosis platform in response to the “sample-to-answer” of IVD (In-Vitro Diagnostics) market trends. OPTOLANE’s core technologies are the integration of CMOS sensors with the fluorescent optical and thermal control systems in order to detect target genes and immunoassay diagnostic platform to the disease diagnosis and preventive home healthcare market. Our new diagnosis systems are small but simple, fast, and smart.

The key to improving cancer survival is early detection and optimal selection for therapy. As a company, we are driven by our passion to improve cancer survival and give people ‘extra time’. Oncimmune’s immunodiagnostic blood test, EarlyCDT Lung, can detect and help identify lung cancer on average four years earlier than standard clinical diagnosis. With over 200,000 tests already performed for patients worldwide, and its use being supported by peer reviewed data in over 12,000 patients, EarlyCDT Lung is poised to become an integral part of future lung cancer detection programs, globally.

At KDx Diagnostics, we believe in the advancement of bladder cancer recognition. Ever since our founding in 2016, we have worked endlessly to achieve the highest sensitivity and specificity test. With the creation of our URO17®️ bladder cancer test, we have achieved an inexpensive and non-invasive assay that is simpler to use for the patient with results that are more accurate than ever before. We hope with the introduction of the URO17®️, we can strive for quicker recognition and response to the dangers of cancer. Our goal is to help patients achieve a peaceful and care-free life.

Immunostep, a leading provider of technologies, tools, and services for bioscience research and biopharmaceutical manufacturing, offers of a range of assays, kits and solution for flow cytometry. Flow cytometry is a powerful method used to measure cell numbers, cell viability, programmed cell death (apoptosis), cell division, toxicity, and the differential expression of specific proteins that can help scientists understand the biology of embryonic development, cancer, metabolic and degenerative diseases, drug effects, and even aging.

Immundiagnostik AG was founded by Dr. Franz Paul Armbruster in 1986 and is now an internationally active diagnostics company with representatives in over 70 countries. The focus of our work is the development and production of innovative immunoassays, LC-MS/MS, HPLC, PCR and other analytical detection methods for medical routine and research. In addition, you will find a wide range of antibodies and antigens in our portfolio. Further services like proficiency testing or contract developments complete our program.

Our core businesses are in the fields of both research and medicine. We research, develop, manufacture and supply various immunological research reagents, such as ELISA kits, antibodies, cell culture-related products, and customized services. We also conduct research and development on the materials used in diagnostic products and valuable seeds in our existing portfolio, and aim to obtain approval for their use in in-vitro diagnostic products and drugs.

The NEW STANDARD in IgE allergy testing.
We are solving challenges faced today in laboratories and clinical practices with the NOVEOS System, the first technological breakthrough in routine allergy diagnostics in 20 years.

Fine Biotech is an ISO9001:2015 Certified Company,offers a full line of quality research kits,which include ELISA Kits,related ELISA assistant reagents and more high quality antibodies, recombinant proteins.

DIAsource ImmunoAssays® S.A. (A BioVendor Group company) is an international diagnostic company based in Belgium with more than 30 years of experience in IVD (kits and instrumentation).
DIAsource’s product range is very comprehensive, ranging from manual assays and instruments for small laboratories (Readers ,Washer, Shakers, Gamma-Counters) to assays and instruments offering full automatization to medium-sized and high- throughput Clinical laboratories (for ELISA Stratec Gemini and Dynex Technologies instruments e.g.DS2®, DSX® and Agility® and for RIA automation CoNext125 sample Preparation Automate).

Creative diagnostics offers a wide range of high quality antibodies validated for use in multiplexed assays. We have primary antibodies to multiple forms of virtually every protein in the proteome including cytokines.

YHLO (EST. 2008) is an innovative and steadfastly growing company of immunoassay solutions headquartered in Shenzhen, China, specialized in developing, manufacturing and distributing in-vitro diagnostic instruments and reagents by a team of experienced scientists and engineers.
In May of 2021, YHLO has successfully launched IPO and listed on Shanghai Stock Exchange (Stock code: 688575).
To be a reliable and respected IVD enterprise, YHLO has put extensive investments in R&D and firmly associated with the top-tier universities, medical schools and research institutions worldwide on scientific studies. With over a decade’s continuous development expertise in immunoassays solution, YHLO may provide a broad range of instruments (based on Chemiluminescence, ELISA, Immunoblot and Immunofluorescnce methodologies) with patented technologies and outstanding features, as well as a comprehensive total solutions with portfolios in fertility and reproductive health, diabetes, infectious diseases, autoimmune disorders and other novel assays on rare diseases analysis.

Blackhills Diagnostic Resources (BDR) was founded in Zaragoza (Spain) in 2012. It´s a Spanish biotechnological company focused in researching, developing, manufacturing and commercializing IVD products.
All the products offered by BDR have been manufactured in its facilities and they are offered not only with BDR brand but also in OEM terms.
BDR has large experience in molecular techniques like Real Time PCR, fragment analysis and sequencing. BDR offers its experience to develop new products in collaboration with other companies.

ABclonal is a dynamic and growing provider of high quality biology research reagents, tools, and custom services. They offer a large selection of Antibodies, Proteins and Peptides, Molecular reagents and ELISA assays. You can find their products in a huge number of Publications world-wide.





